
Immunosensor for Detection of Inflammation in Amniotic Fluid
A point-of-care test based on electrochemical immunosensor for simultaneous determination of three significant inflammatory markers.

A point-of-care test based on electrochemical immunosensor for simultaneous determination of three significant inflammatory markers.
Preterm Premature Rupture of Membranes (PPROM) is a pregnancy complication. In this condition, the sac (amniotic membrane) surrounding the fetus breaks (ruptures) before week 37 of pregnancy. Once the sac breaks, pregnant woman has increased risk for infection. PPROM complicates 3 – 4% of all pregnancies and is up to 1/3 complicated by microbial invasion of the amniotic cavity (MIAC) leading to infection in amniotic fluid (AF) and development of intra-amniotic inflammation. Although this complication is usually asymptomatic, it is a major cause of preterm birth and neonatal morbidity and mortality worldwide. Neonates from these pregnancies are at increased risk of developing neonatal sepsis and impaired psychomotor development and other sometimes lifelong health consequences.
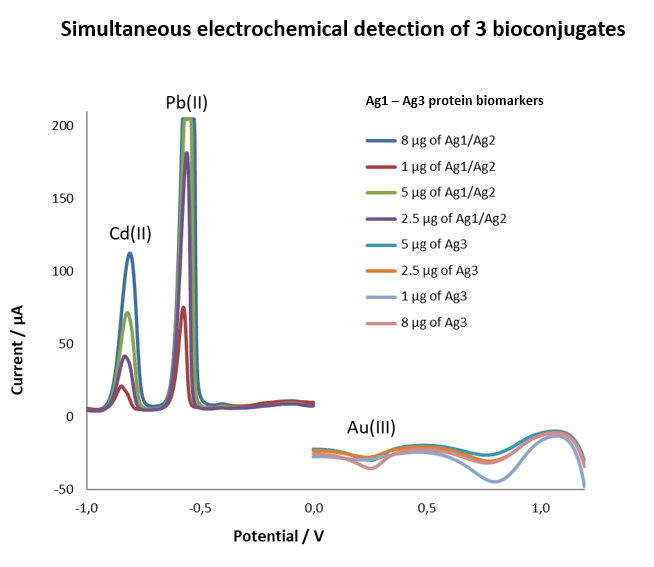
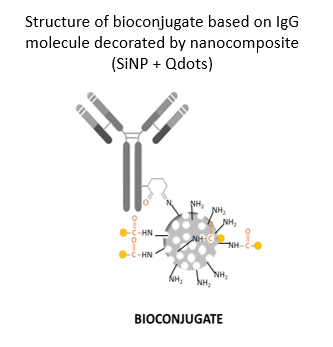
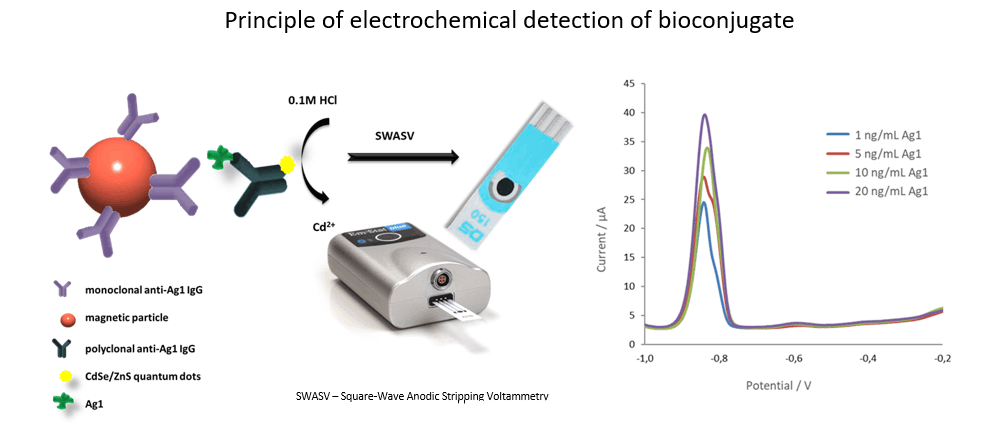
Our invention is point-of-care test (POCT) based on electrochemical immunosensor for simultaneous detection of three inflammatory protein biomarkers with high predictive value in AF collected by amniocentesis. The aim of the test is to confirm intra amniotic inflammation. The entire biosensor consists of an immunosorbent magnetically active microparticles modifed with specific antibody. After capturing the protein biomarker by the immunosorbent, the bioconjugate (IgG antibody conjugated with an electroactive indicator, specifically quantum dots or gold nanoparticles) is added. A final measurable signal proportional to the concentration of the inflammatory protein biomarker is provided by metal ions released from the electroactive indicator. Disposable screen printed three electrode sensors are used for simultaneous electrochemical analysis. Clinical relevance of selected protein biomarkers was determined based on the results of proteomic and antibody studies of AF of pregnant women with PPROM.
Advantages
POCT enables to speed up the process of confirmation or elimination inflammation in AF and can be easily performed and evaluated in minutes beside the patient´s bed and thus enables personalised approach to therapeutic intervention of the pregnant woman. Currently, determining the MIAC is not specific enough and is very time consuming and technically demanding. It is based on combination of cultivation and molecular methods. Further research is focused on test verification in cervicovaginal fluid to avoid amniocentesis.
POCT detecting multiple inflammatory markers at the same time with high predictive value.
Acceleration of the process of confirmation / elimination of the type of inflammation in amniotic fluid – performance and evaluation of the test ina matter of minutes beside the patient’s bed.
Personalized approach to therapeutic intervention of the pregnant woman based on the results of the examination.
The experience of the research team, which has been systematically involved in PPROM problematic since 2008 and is one of the most productive team in this field, not only in the European context.
Technology is owned by University Hospital Hradec Králové together with University of Pardubice. Czech patent application PV 2021-401 (priority date 31/08/2021) and PCT application PCT/CZ2022/050080 (priority 21. 08. 2022) was submitted. Technology is currently going through Proof of Concept stage of development. Potential application is IVD test - POCT is intended to be used my medical facilities that take care of pregnant women esp. regional hospitals or perinatology centers.
Chief physician of the Perinatology Center
Chairman of the Section of Perinatology and Fetomaternal Medicine of the Czech Gynecological and Obstetrical Society
Author & coauthor of more than 150 publications in prestigious international journals
Molecular biology and genetics, immunochemistry
Investigator & coinvestigator in multiple international studies and projects (FP5, FP6, Horizon 2020, GAČR, TAČR)
Author & coauthor of more than 100 publications in prestigious international journals
For more detailed information, do not hesitate to contact us directly:
Lucie Bartošová, Ph.D., lucie.bartosova@fnhk.cz or +420 495 832 925.
Stay in touch with us on social media where you can follow recent progress in developing technologies.
Mobirise.com