Bacterial Detection in Amniotic Fluid
Determination of the presence of specific bacteria in amniotic fluid collected by amniocentesis using multiple RT-PCR test.

Determination of the presence of specific bacteria in amniotic fluid collected by amniocentesis using multiple RT-PCR test.
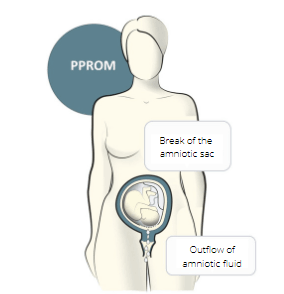
Preterm Premature Rupture of Membranes (PPROM) is a pregnancy complication. In this condition, the sac (amniotic membrane) surrounding the fetus breaks (ruptures) before week 37 of pregnancy. Once the sac breaks, pregnant woman has increased risk for infection. PPROM complicates 3 – 4% of all pregnancies and is up to 1/3 complicated by microbial invasion of the amniotic cavity (MIAC) leading to infection in amniotic fluid (AF) and development of intra-amniotic inflammation. Although this complication is usually asymptomatic, it is a major cause of preterm birth and neonatal morbidity and mortality worldwide. Neonates from these pregnancies are at increased risk of developing neonatal sepsis and impaired psychomotor development and other sometimes lifelong health consequences. Currently, determining the MIAC in patients with PPROM is very time-consuming and technically demanding. Main disadvantage is that results are available in days, which is already clinically irrelevant for the initiation of targeted antibiotic treatment.
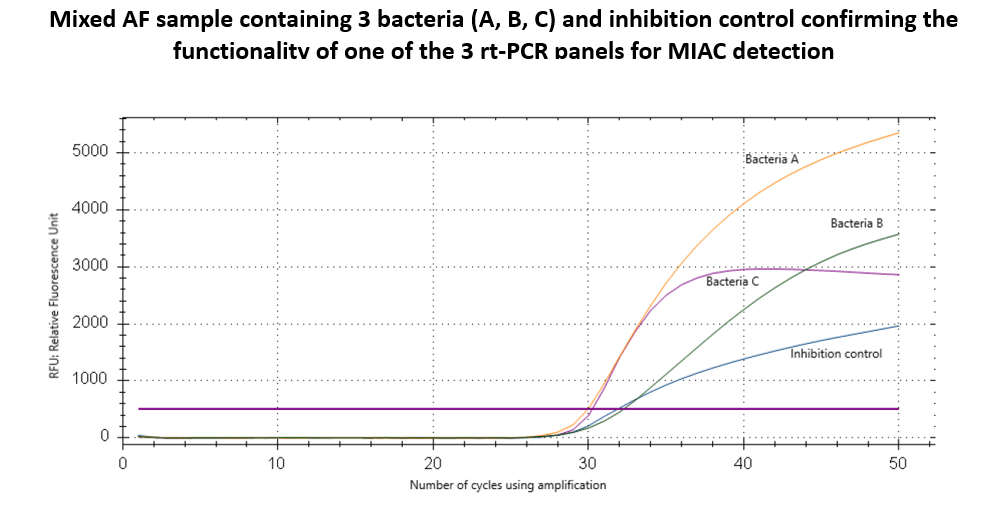
We have developed multiplex Real Time - PCR (Real - Time Polymerase Chain Reaction) assay for simultaneous detection of the most common targeted pathogens in AF collected by amniocentesis. The method will reduce the time required to diagnose specific pathogen and help clinicians make quick and accurate treatment decision. The assay consists of 3 panels for DNA detection of 8 most common microorganisms. Each panel contains specific sets of primers and probes. Assay results are determined by PCR fluorescence signal. Panels were validated retrospectively on 20 samples of AF of PPROM patients and have reached 90 % sensitivity so far. Further prospective validation study is currently underway.
Advantages
High sensitivity of the method - panel of selected microorganisms in multiplex assay was defined on the basis of research in nearly 700 patients with PPROM and it covers 88 % of microorganisms responsible for MIAC in PPROM patients. The multiplex approach determines the selected microbes in several hours after sampling in semi-quantitative way compare to the current approach (combination of specific multiplex Real Time - PCR for 3microorganisms in combination with PCR assay targeting 16S rRNA regions followed by Sagner sequencing for the rest of targeted microbes and cultivation techniques). Personalized approach to the clinical management and therapeutic intervention of the patients with PPROM based on the assay results. The technology does not require specialized laboratory equipment or specialized personnel and thus is suitable for molecular laboratory of perinatology centres equipped with standard Real-Time PCR cycler.
High sensitivity of the method – 88 % of pathogens.
Time of evaluation – within hours after sampling, on top of that it is usable for perinatology centers equipped with a PCR cycler (does not require specialized laboratory or personnel).
Personalized approach to therapeutic intervention of the pregnant woman based on the results of the examination.
The experience of the research team, which has been systematically involved in PPROM problematic since 2008 and is one of the most productive team in this field, not only in the European context.
Technology is owned by University Hospital Hradec Králové and is being investigated within the PERSONMED project (CZ.02.1.01/0.0/0.0/17_048/0007441). Intellectual property is protected with know-how. Technology is currently going through Proof of Concept stage of development. Potential application is IVD test - POCT is intended to be used my medical facilities that take care of pregnant women esp. regional hospitals or perinatology centers. Our final product is planned to be IVD test (guideline EU 2017/746). We are searching for partner - IVD manufacturer (Generi Biotech, Biovendor, Genespector, Roche).
Chief physician of the Perinatology Center
Chairman of the Section of Perinatology and Fetomaternal Medicine of the Czech Gynecological and Obstetrical Society
Author & coauthor of more than 150 publications in prestigious international journals

Specialist in laboratory methods
Member of the Society for Medical Microbiology
Coauthor of more than 10 publications
Coinvestigator of 5 project
For more detailed information, do not hesitate to contact us directly:
Lucie Bartošová, Ph.D., lucie.bartosova@fnhk.cz or +420 495 832 925.
Stay in touch with us on social media where you can follow recent progress in developing technologies.
Free AI Website Creator