Multiple Myeloma
Blood-based highly sensitive diagnostics of multiple myeloma and monitoring of its progression.

Blood-based highly sensitive diagnostics of multiple myeloma and monitoring of its progression.
Background
• Multiple myeloma (MM) = cancer of plasma cells (PC) that produce monoclonal immunoglobulins (mAb, M-protein).
• Disease complications = high likelihood of developing persistent minimal residual disease (MRD) and relapse, both of which are associated with the presence of drug-resistant malignant plasma cell clone(s) - primary or emerged during treatment through clonal heterogeneity and evolution.
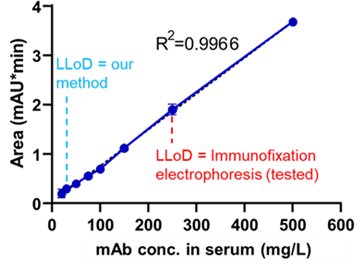
• Detection of minor and persistent clones from blood is currently limited = low sensitivity of current electrophoretic serum-based methods x more sensitive methods requiring invasive bone marrow biopsy.
• Main objective = to develop a simple and widely applicable method for sensitive detection of malignant PC clones based on antibody analysis in human serum. This method will allow early and sensitive detection of malignant clone progression.
Our Method
• Isolation = selective affinity isolation of antibodies from a blood sample using ≤3 different affinity matrices.
• Analysis = separation and detection using conventional HPLC system with UV-VIS detector.
• Validation samples = 5 different mAb standards + 28 patient serum samples (pre and post treatment).
• Possible upgrade = automation of isolation procedure + sensitivity enhancement via mass spectrometric detection of clonotypic peptides.
Conclusion
This novel approach allows highly sensitive and specific identification of primary myeloma cell clones in blood, those that become resistant and require following treatment as well as novel newly emerged clones. Additionally, the method enables distinguishing between pathological and therapeutic antibodies. The method employs a common analytical instrument, thus facilitating its widespread use. The method has a potential to replace standard immunofixation electrophoresis, avoid invasive and problematic bone marrow aspiration, and by that to facilitate early detection of disease relapse due to possibility of close patient monitoring at brief intervals.
Technology is owned by University Hospital Hradec Králové and is protected as an industrial secret.
Technology is going through Proof-of-Concept stage of development.

Biomedical Research Centre
For more detailed information, do not hesitate to contact us directly:
Lucie Bartošová, Ph.D., lucie.bartosova@fnhk.cz or +420 495 832 925.
Stay in touch with us on social media where you can follow recent progress in developing technologies.
Website Building Software